Introduction to Our Research
Motivation
Cold Molecules
Isolated molecules are ideal systems to test our understanding of fundamental physics. Due to their relative simplicity, we can calculate some of their properties to extreme levels of accuracy. If such predictions agree with the results of experimental measurements, we must conclude that our understanding is correct---at least to that level of accuracy. Determining molecular structures or the distributions of electronic charge in molecules, for example, is important for the development of chemistry. However, molecules are also good systems to test our understanding of nature in a much broader sense. Some properties of the world are fixed by physics derived from mathematical symmetries, while others seem to be selected from an ensemble of possibilities. On the one hand, it is interesting to know whether our theories are correct and which mathematical symmetries should hold. For example, time reversal symmetry is when the laws of quantum mechanics are left unchanged if time runs backwards. One of the consequences of this symmetry is that fundamental particles cannot show an electric dipole moment. So, finding a non-zero electric dipole moment in, for example, an electron would force us to review our theories. It turns out that the accurate measurement of some molecular states is one of the best ways to determine the magnitude of such a dipole moment. On the other hand, the values of some fundamental physical constants are extremely critical in shaping the world as we know it. A small change in some values and life on our planet would not be possible as we know it. It is natural to wonder about the reasons for these specific values and how constant these constants really are. Since the comparison of two measurements separated by a million years is unpractical, it is better to improve the accuracy of a single measurement by one million times and repeat it after one year. Again, molecules are ideal systems for these kinds of experiments.
Ultra-cold molecules are appealing also for quantum simulators instead of atoms. Quantum simulators are synthetic systems whose Hamiltonian is similar to that of a real physical system but is too hard to calculate accurately. So, for example, instead of studying electrons flowing in the lattice of a metal, we study atoms in an optical lattice. If we replace the confining potential of positively charged nuclei in metals with electric field maxima in an optical lattice, the latter force can be easily tuned by tuning the intensity of the laser creating the lattice. The advantages of using molecules are mainly threefold. First, the electric dipole-dipole interaction between molecules can have a much longer range than the magnetic dipole-dipole between atoms. Second, such interaction can be tuned with external fields. Third, the interaction in anisotropic, which adds further degrees of freedom to the simulator.
Experiment
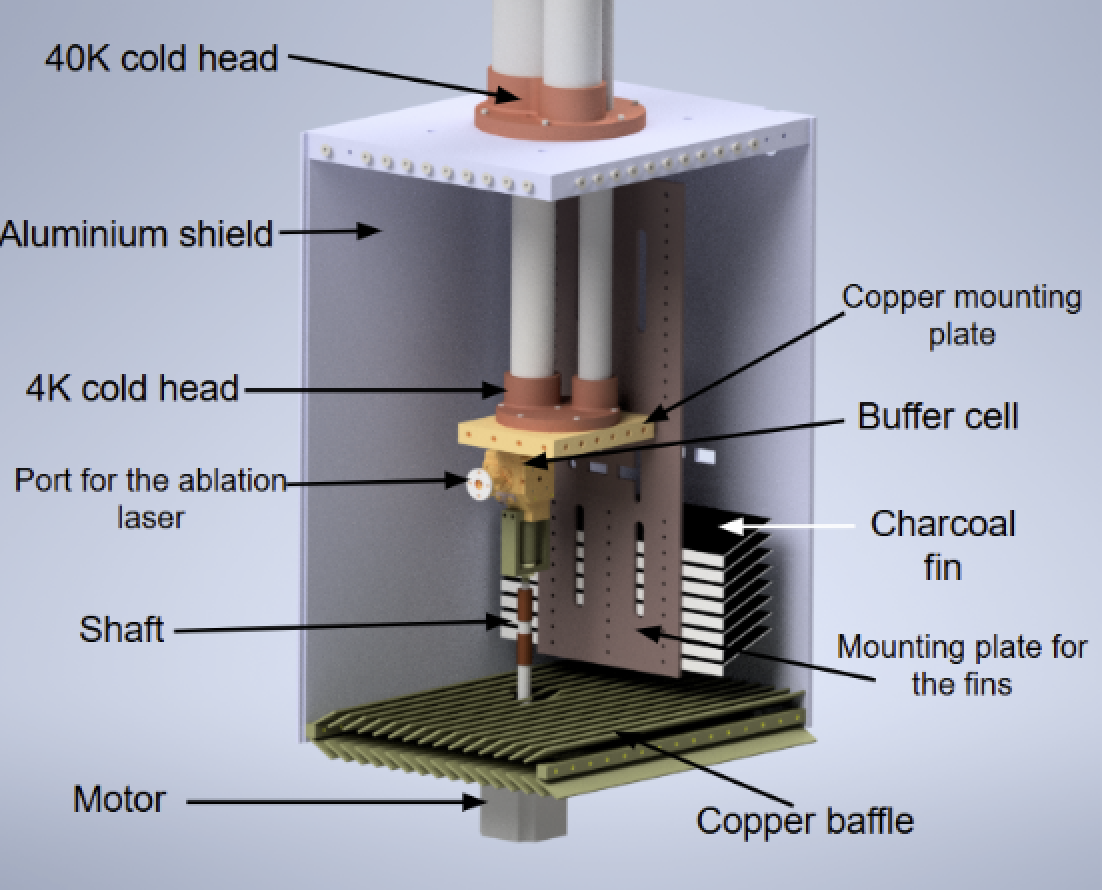
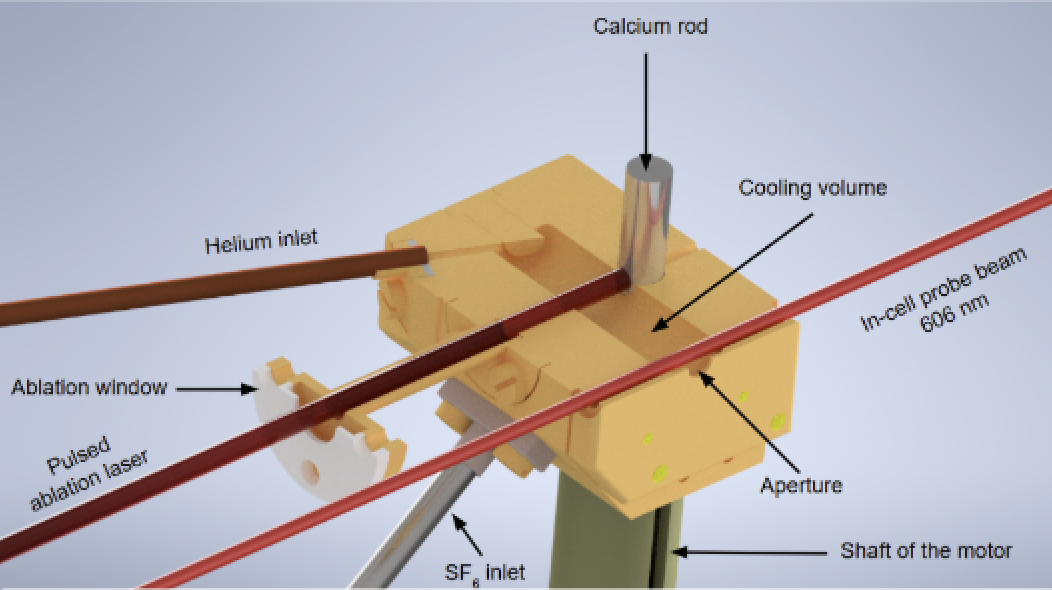
We work with CaF molecules. They are produced in a buffer gas cell. This is a small copper cell cooled down at 4 K and filled with He gas. A laser pulse evaporates some calcium from a calcium rod, creating a plasma. The plasma reacts with some SF6 gas, also present in the cell, and some CaF molecules are produced. Molecules escape the cell from a hole and form a beam in the vacuum chamber. The low temperature of the cell helps to keep the velocity of the beam relatively low, at around 160 m/s.
The CaF beam is first slowed with resonant green light and then some molecules are trapped in a magneto-optical trap (MOT). Currently we are improving the slowing part and soon we will test our MOT.